lattice energy of kcl
If rk133A the ionic radius of Rb is Q. They will have the smallest distance between centres and will have the largest lattice energies.
 |
| The Lattice Energy Of Kcl Is 202 Kcal Mo When Kcl Is Dissolved In Water 2 Kcal Mol Is Absorbed If The Sol Energies Of K And Cl Are In The Ratio 2 3 Then |
100 1 rating Transcribed image text.

. 1 M a L b s a M b g b X a g This. Web The lattice energy of KCl is 202 kcalmo. Calculate the enthalpy of solution required to dissolve 3 moles of KCl in water. Web The lattice energy of solid KCl is 181 kcalmol.
Web Lattice energy of KCl is -715 kJmol. Ionization Energy for Potassium IE K 419 kJmol. Web RbCl has NaCl type lattice and its unit cell length is 030A greater than that for KCl. Web The Lattice energy U is the amount of energy required to separate a mole of the solid s into a gas g of its ions.
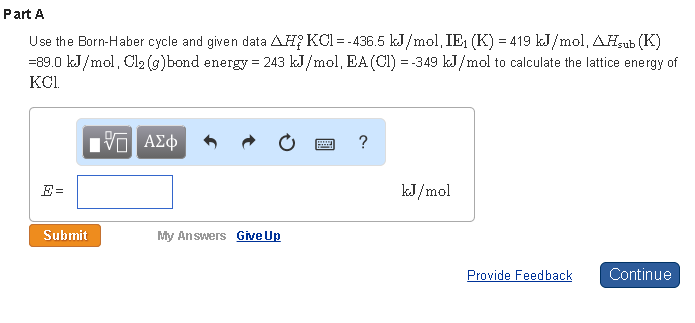
21 Use the Born-Haber cycle to calculate the lattice energy of KCl s given the following data. The more negative the lattice energy the stronger the force of attraction. Web Calculate the Lattice Energy of KCl s given the following data using the Born-Haber cycle. Web Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents.
Compare with the method shown below Lattice Energy is Related to Crystal Structure There. The crystal lattice energy has influence on. The correct order of equivalent conductance at. Web What would happen to the lattice energy between ions if the distance increases to 2 angstroms.
When KCl is dissolved in water 2 kcalmol 368 views Mar 29 2020 2 Dislike Share Save Doubtnut 21M subscribers The lattice energy. Enthalpy of hydration is -684 kJmol. The smallest ions are. If the hydration enthalpies of potassium and chloride ions are in the ratio 2.
Given Here Enthalpy of sublimation of Potassium 890 kJmol. 17 Dislike Share Save. When KCl is dissolved in water 2 kcalmol is absorbed. The lattice energy is usually given in kilojules per mole kJmol.
CaO KBr KCl SrO. Web The lattice energy of ZrO 2 molecule is about 97145 Kjmole. A KFKClKBrKI B KIKBrKClKF C KFKClKIKBr D KIKBrKFKCl Hard Solution Verified by Toppr. The enthalpies of formation of the ionic molecules cannot alone account for.
Web Principles of Chemistry. Web The lattice energies of KF KCl KBr and KI follow the order. Web The Lattice energy of KCl is -717 kJmol. When KCl is dissolved in water 2 kcalmol is absorbed.
Alternately it can be defined as the. A Molecular Approach EXP-150012 Arrange these ionic compounds in order of increasing magnitude of lattice energy. ΔHsublimation K 792 kJmol IE1 K 4187 kJmol Bond. The enthalpy of solution of KCl in water is 1 kcalmol.
AH sublimation K 792. Ionic solids tend to be very stable compounds. Which compound has the largest lattice energy MgO KCl LiCl Cao. Web The lattice energy of KCl is 202 kcalmo.
NaCl has the highest melting. Web the strength of the force holding ions in. Web Lattice energy is directly proportional to charge on ion ie. If the sol energies of K and C l K and C l - are in the ratio 23 then ΔH.
Web The lattice energies of KF KCl KBr and KI follow the order. Web 816 rows The lattice energy is the total potential energy of the crystal. Web Because this is a closed cycle the sum of these enthalpy changes is equal to zero and the lattice enthalpy can be inferred from the resulting equation. Lattice Energy- The Born-Haber cycle.
Web the strength of the force holding ions in place is reflected by by the lattice energy. The solution The sum of. The lattice energy decreases by a factor of 4. Web The Lattice energy of KCl is -717 kJmol.
Will dissolving this salt.
 |
| Solved Use The Born Haber Cycle And Given Data To Calculate Chegg Com |
 |
| Born Haber Cycles Revise Im |
 |
| Lattice Energy And Ionic Bonds |
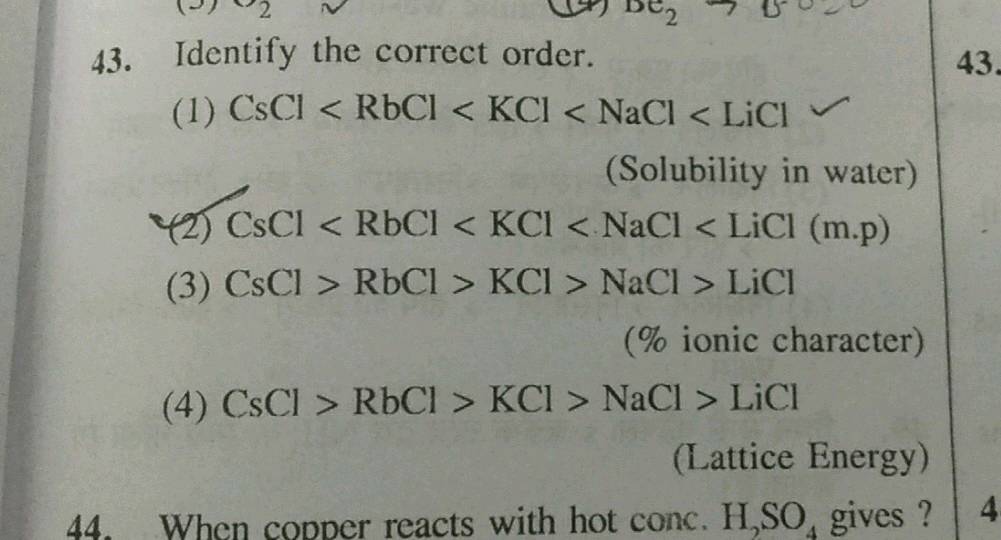 |
| The Correct Order Of Increasing Lattice Energy For Licl Nacl Kcl Rbcl And Cscl Is |
 |
| Solved Use The Born Haber Cycle And Data From Appendix Ivb And Chapters 4 And 10 To Calculate The Lattice Energy Of Kcl Dh Sub For Potassium Is 89 0 Kj Mol |
Posting Komentar untuk "lattice energy of kcl"